Abstract
Background: Castleman disease (CD) is a heterogeneous rare disorder, characterized by lymph node enlargement with a common histopathological spectrum. This disease comprises the subtypes unicentric (UCD), HHV8-associated multicentric CD (HHV8 MCD), POEMS-associated MCD (POEMS MCD) and idiopathic multicentric CD (iMCD). Whereas UCD is a localized reversible disease, the other three subtypes are systemic diseases with multiple lymphadenopathies. The TAFRO (thrombocytopenia, anasarca, fever, reticulin myelofibrosis, organomegaly) syndrome is an aggressive form of iMCD. Each CD subtype has different clinical features, prognosis and treatment.
Aims: To study a large series of patients diagnosed with CD to describe the clinical and biological characteristics, as well as the treatments and outcomes of the different CD subtypes.
Methods: Multicentric retrospective study which includes patients diagnosed with different subtypes of CD in 20 Spanish hospitals from 2008 to 2022. Clinical and biological data were retrieved from the clinical records. The project was approved by the Ethical Committee of Germans Trias I Pujol Hospital (PI-20-103). Statistical analyses were performed using SPSS v24.0 (IBM, Somer, NY).
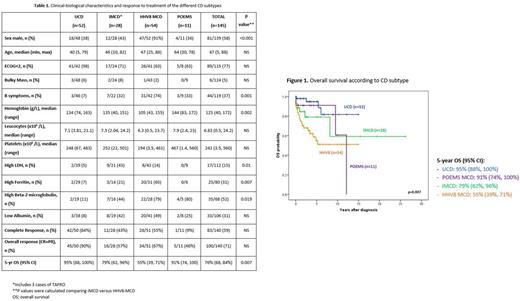
Results: One hundred and forty-five patients with available data were included; 52 with UCD and 93 with any of the 3 subtypes of MCD. The median follow-up was 3.4 years. The main clinical and biological characteristics of the 4 CD subtypes are shown in table 1 and differences in OS in figure 1. Most patients with HHV-8 MCD were HIV-infected (74%), whom 78% had positive HIV load (median [range]: 85603.5 copies/mL [227, 10000000]); with median CD4 lymphocyte count 0.209x109/L (range: 0.016, 1.2) and 48% of them had concomitant or prior Kaposi's sarcoma. Three iMCD patients had TAFRO. All patients with UCD were treated with surgery. Patients with HHV-8 MCD were treated at front-line with rituximab +/- doxorubicin +/- antiviral (63%) or other treatments such as polychemotherapy +/- rituximab or steroids (37%). Patients with iMCD were treated with anti-IL-6 (27%), polychemotherapy plus rituximab (20%), rituximab alone (33%) and steroids (20%). Treatment of POEMS MCD was also diverse; 3 patients received lenalidomide plus dexamethasone, 2 polychemotherapy, 1 anti-IL-6 and 1 auto SCT (3 patients unrecorded). The results of the first-line treatments administered in each CD subtype are shown in Table 1. Eleven patients (7.6%) had concomitant or evolved lymphoma (2 UCD, 2 iMCD and 7 HHV-8 MCD). Thirty-four patients are dead (21 HHV8-MCD, 6 iMCD-TAFRO, 4 UCD, 3 POEMS), 7 of them from CD (5 HHV8-MCD, 1 iMCD-TAFRO, 1 UCD).
Conclusions: Castleman Disease subtypes have different characteristics and outcomes. First-line treatment is heterogeneous in all MCD subtypes. Patients with CD have an increased risk of developing lymphoma being higher in HHV8-MCD.
Disclosures
Navarro:EUSA Pharma: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; BluePrint Medicines: Consultancy; GILEAD: Research Funding. Gonzalez Barca:Novartis: Consultancy; Beigene: Consultancy; Lilly: Consultancy; Kiowa: Consultancy; Gilead: Consultancy; Incyte: Consultancy, Honoraria; EUSA Pharma: Honoraria, Other: travel, accommodations, expenses; Roche: Honoraria; Takeda: Honoraria; AbbVie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses. Climent:EUSA-Pharma: Speakers Bureau. Bastos-Oreiro:JANSSEN: Speakers Bureau; INCYTE: Consultancy, Speakers Bureau; NOVARTIS: Speakers Bureau; KITE/GILEAD: Consultancy, Honoraria; Roche: Consultancy, Research Funding, Speakers Bureau. Abrisqueta Costa:AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Abbvie: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers-Squibb: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau; Sandoz: Speakers Bureau. Ocio:GSK: Research Funding; Amgen, BMS/Celgene, GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Sanofi, Takeda: Consultancy; Pfizer: Consultancy, Honoraria; Oncopeptides: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy; GSK: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen, BMS/Celgene, GSK, Janssen, Oncopeptides, Pfizer, Sanofi, Takeda: Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Janssen, Takeda: Speakers Bureau. Fernández De Larrea:Pfizer: Honoraria; Beigene: Consultancy, Honoraria; Sanofi: Consultancy; GSK: Honoraria; Takeda: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Pérez Ceballos:Roche: Honoraria; Incyte: Consultancy; Janssen: Consultancy; Takeda: Consultancy, Honoraria. Hernández-Rivas:Takeda: Speakers Bureau; Rovi: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Lilly: Membership on an entity's Board of Directors or advisory committees. García-Sanz:Janssen: Honoraria, Other: Travel support, Research Funding; BeiGene: Honoraria, Other: Travel Support; Gilead: Honoraria, Research Funding; Astellas: Honoraria, Research Funding; Amgen: Honoraria; Takeda: Honoraria, Research Funding; GSK: Honoraria, Other: Travel Support; Astra Zeneca: Honoraria; In Vivo Scribe: Patents & Royalties: Indirect perception, Euroclonality primers; Novartis: Honoraria, Research Funding. Sancho:Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria; Eli Lilly & Company: Consultancy, Membership on an entity's Board of Directors or advisory committees; Miltenyi Biomedicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kern Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal